
By partnering with many of the world’s leading cell suppliers, we are able to access the most suitable cells for each and every battery pack that we produce. As a result of our independence and the range of cell options at our disposal, we can fully optimise our battery packs to perfectly suit the application.
We have access to cells covering the most commonly used chemistries from Lithium-ion (Li-ion) to Nickel Metal Hydride (NiMH) and Alkaline to Lithium Thionyl Chloride (Li-SOCI2), so whatever battery pack chemistry is best suited to a given application, we can produce it.
This type of battery is termed as secondary and refers to a battery that can be used many times by recharging when depleted. Typically, these batteries power equipment for hours to days at a time but rarely longer. If your application needs long term power spanning months or years, you will most likely need a single use primary battery.
Choosing the correct chemistry type is a key part of the service Custom Power offers, lithium ion is not the only solution nor necessarily the best solution for your application. As part of understanding your products energy demands, we can help agree the right battery to power it. We are not tied to any supplier or cell type, and so as a customer of Custom Power, you can rest assured that we can and will choose the very best cell option for your requirements.
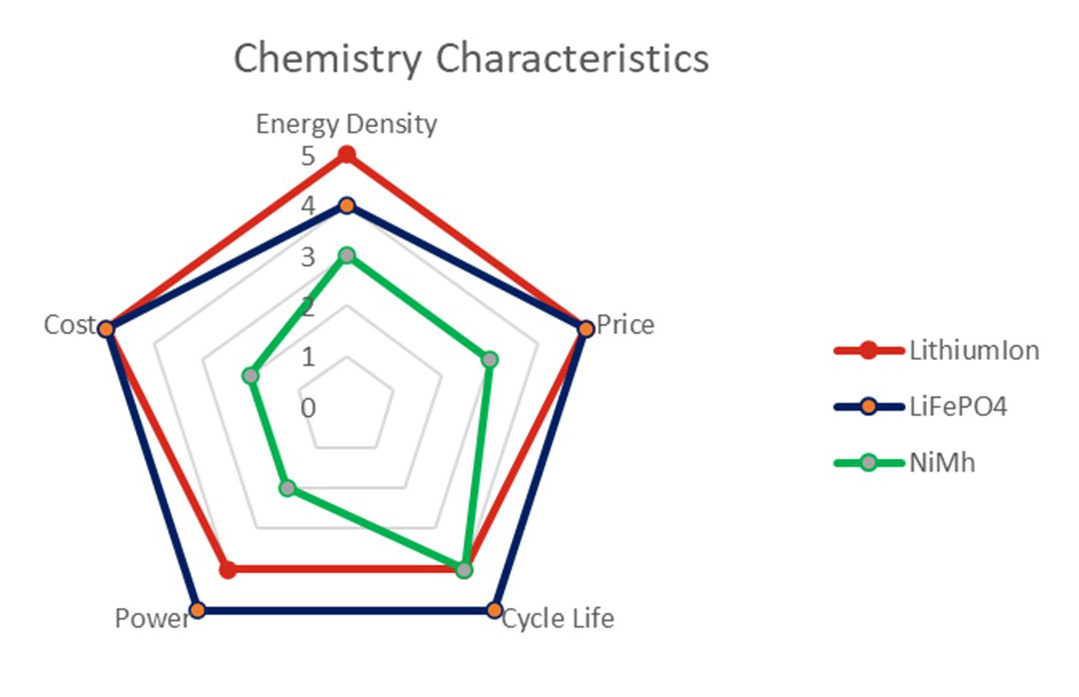
This is currently the most requested rechargeable chemistry and has been available for almost thirty years. These cells offer the most energy dense solution of the currently available types, measured both by mass (watts per kilogram) and volume (watts per cubic metre).
Pros: This chemistry provides high power delivery, fast charge times and low self-discharge (power lost when battery is charged but providing no energy to the equipment).
Cons: The price of cells and therefore final battery packs is relatively high. The development time for bespoke battery packs also tends to be longer and there are stringent transportation test requirements for certifying packs as safe to use.
Li-ion packs come in assorted shapes and sizes. Primarily there are three formats, cylindrical cells, prismatic cells and pouch cells.
Pouch cells are typically used for applications that need the battery in a particular shape, or where the highest energy density (energy within a given volume) is sought. Examples include smart watches, tablet computers and battery powered electric vehicles. A drawback of pouch cells is they are prone to swelling particularly as they age which leads to rapid loss of capacity and failure. In larger applications they are frequently contained in strong structures to keep them compressed to avoid this swelling.
Cylindrical cells are the most commonly produced format and offer similar energy density.
Also termed Lithium Ferro-phosphate (LFP), or lithium nano phosphate (LiFePO4), this is a more recently developed lithium-based chemistry but still proven and developed in the 1990’s. It has slightly less energy density than li-ion, around 15% reduction however it is considered a safer chemistry and offers exceptional power output and life. Batteries will last for thousands of charge/discharge cycles versus hundreds of cycles for li-ion.
Pros: This chemistry offers very high-power delivery, very fast charge times, low self-discharge, and is the safest lithium ion-based chemistry.
Cons: As with lithium ion, lithium iron phosphate has a relatively high price, longer development time for bespoke battery packs, and again there are transportation test requirements for certifying packs as safe to use. Lithium iron phosphate also has a lower energy density than competing li-ion types.
This is a widely available and commonly used rechargeable chemistry type. This was developed as an alternative and ultimately as a replacement for Nickel-Cadmium (NiCad) chemistry cells.
Nickel metal hydride (NiMh) offers more capacity than the NiCad and uses more readily available and less harmful materials. The technology is deemed safer than li-ion and requires less protection devices (electronics) to be used in the design. It does not require transportation testing which saves time and expense.
The cell is often used for lower power consumption applications, small scale backup power and where standard size single use cells are commonly used, in applications such as torches and remote control units for instance. A large number of hybrid powered vehicles use NiMh as the energy store, notably Toyota in their Prius model.
Pros: NiMh offers good power delivery, tolerates a wide operating temperature, and is less complex and expensive as lithium alternatives.
Cons: NiMh offers lower specific energy than batteries that use lithium cells, and also tends to generate more heat during charging cycles. The chemistry also suffers from relatively high self discharge rates and tolerates fewer charging cycles that lithium based cells.
Unlike secondary (rechargeable) batteries, primary batteries are designed to be used just once and then replaced with a fresh battery. Alkaline batteries are the most widely recognised of this type, but there are a number of chemistries in use depending on the requirements of the end application.
In primary cells, the electrochemical reaction cannot be reversed as it can in secondary cells, and as such batteries that use primary cells cannot be recharged. Although generally more expensive across their lifetime, primary batteries do have a number of benefits over their secondary counterparts. They have higher specific energy than rechargeable types and can be stored for longer with lower self discharge rates. In addition, they offer instant readiness for situations where recharging is not viable.
Mainly seen in usual consumer channels, this is often marketed at ‘Ultimate Lithium’ or similar and available in AAA and AA formats. These were created in 1989 to satisfy the growing need for more capacity for electronic products to increase runtime or provide more power. The cells are more expensive, less heavy and a flatter discharge curve – the voltage stays broadly the same throughout the cells discharge. In simple terms, they have approximately twice the capacity of a good alkaline cell of the same size.
These are the most commonly available and well-known single use primary type of battery cell. Made from a combination of zinc and manganese dioxide, Alkaline cells account for a large proportion of all cells manufactured in the world at this time, and until the growth in lithium-ion recently, was comfortably the largest share of the market.
The battery is inexpensive and primarily used in consumer products in familiar formats, the AAA, AA, C, D and PP3 shapes. The cells are all 1.5V (PP3 is actually 6 x 1.5 cells inside a can), have good low self-discharge and may be stored for up to 10 years before being used. Whilst they have considerably more capacity than the older Zinc Carbon type batteries, they are still comparatively poor in energy density compared to newer lithium chemistries.
The cells are generally considered very safe, easy to handle and are a proven and well understood technology for powering low energy requirement equipment. The cell has an alkaline electrolyte – potassium hydroxide, which gives the chemistry its name. When exposed to air, this reacts with the carbon dioxide to form potassium carbonate, the white crystalline substance found when a battery has been depleted and left in situ for a long period, Potassium carbonate is corrosive.
Energizer and Duracell are both well know examples of Alkaline battery manufacturers.
This cell is no longer commonly found, it has relatively low storage life and very low capacity particularly in continuous or high energy applications. Again, it was found in the common AAA, AA, C, D and PP3 formats and is sometimes still available at very cheap prices, but this reflects the poor performance.
Interestingly well-known manufacturers of Alkaline cells often tout their products as lasting up to 8 times longer than competitor products, they are actually comparing them to the Zinc-Carbon products so not a like for like valid comparison.
Lithium Sulfur Dioxide as produced by our partner SAFT. These cells operate at 2.8V nominal and tolerate temperatures in the range of -60C to +70C. Because of their ability to operate in extremes of temperature, along with the fact that they can be used in any orientation, offer good pulse power and can be stored for long periods before use, they are ideal for use in applications such as defibrillators, metering, sensing, monitoring and alarm systems.
Lithium Thionyl Chloride. Produced by companies such as Tadiran / Saft / Electrochem / EP (see our partners page for further information on these companies).
Lithium Sulphuryl Chloride. Electrochem is an example of a manufacturer of this type of chemistry.
Lithium Manganese dioxide (Li-Mn). These are found in CR range (coin cells) in 3V variants.
We are well placed to help with your battery requirements, no matter how complex.
With our extensive knowledge of all things battery related, whether cell chemistry, control electronics, enclosures, testing or transportation, and our ability to access a huge range of cells from well respected global manufacturers, we can provide the right solution for you.